Summary:
Long before oxygen became abundant on Earth, ancient bacteria found a way to generate energy through a unique form of respiration.
A study published in Nature reveals how these microorganisms convert carbon dioxide and hydrogen into acetic acid while producing ATP – a key molecule for cellular energy. Scientists at Goethe University Frankfurt identified the role of the Rnf complex, a protein assembly embedded in bacterial membranes, which facilitates electron transfer and pumps sodium ions outside the cell. When sodium ions re-enter, they drive a molecular turbine that powers ATP production.
By using cryo-electron microscopy, researchers captured a detailed view of the Rnf complex in Acetobacterium woodii, showing how its structure enables this energy process. Molecular simulations further explained how an iron-sulfur cluster acts as a switch, attracting sodium ions and opening a pathway for their release. Beyond its biological significance, this mechanism could have industrial applications, including CO2 removal from emissions and potential use in biotechnology. Understanding this ancient enzyme may also inspire new drugs targeting similar respiratory systems in pathogens.
Without oxygen: How primordial microbes breathed
Animals, plants and many other living organisms inhale oxygen to “burn” (technically: oxidize) compounds like sugar into CO2 and water – a process during which the energy-rich molecule ATP is produced. Cells require ATP to power vital reactions. In the early phase of our planet’s existence, however, the earth’s atmosphere did not yet contain any oxygen. Nevertheless, studies of ancient bacteria that still occur today in ecosystems without oxygen, e.g. in hot springs at the bottom of the ocean, suggest that a special form of respiration could have existed even then.
These microorganisms “respire” carbon dioxide and hydrogen into acetic acid. The metabolic pathway with which they do so has been known for some time. The question that remained unanswered until now is how they use this process to produce ATP. The current study now provides an answer.

“We were able to show that the production of acetic acid itself activates a sophisticated mechanism as part of which sodium ions are pumped out of the bacterial cell into the environment,” explains Prof. Volker Müller, Chair of Molecular Microbiology and Bioenergetics at Goethe University Frankfurt. “This reduces the sodium concentration inside the cell, whereby the cell envelope acts like a kind of dam for the ions. Once this dam is opened, the sodium ions flow back into the cell, driving a kind of molecular turbine that generates ATP.”
Cell respiration enzyme isolated just a few years ago
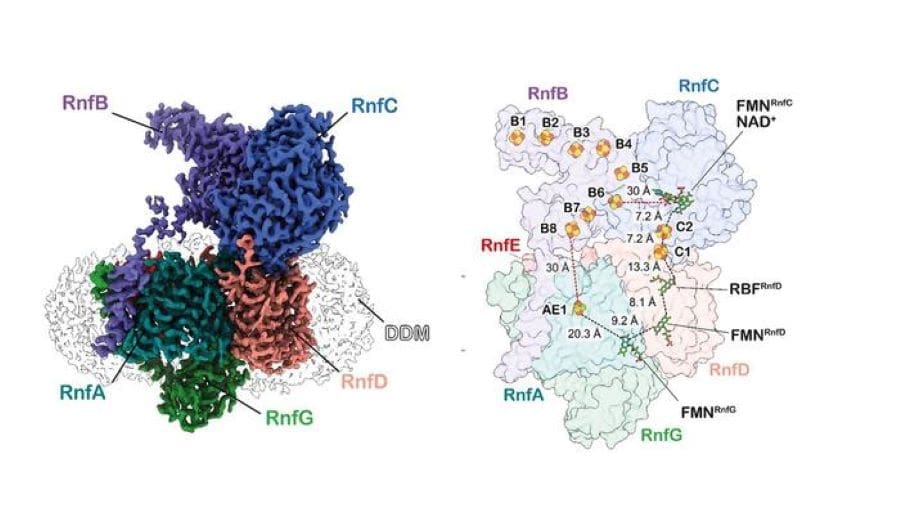
A conglomerate of different proteins known as the Rnf complex plays a key role in this process. These proteins are largely embedded inside the membrane surrounding the bacterial cell. “The complex is so sensitive that we were only able to isolate it a few years ago,” Müller emphasizes. When carbon dioxide reacts with hydrogen to form acetic acid, electrons are transferred from the hydrogen to the carbon atom via a series of different intermediate steps, in which the Rnf complex plays a mediating role: it takes up and passes on the electrons.
In the current study, the scientists have now shown what exactly happens during this process. Structural biologist Anuj Kumar – a PhD student in both Müller’s research group as well as that of Dr. Jan Schuller at the University of Marburg – used a sophisticated method known as cryo-electron microscopy, as part of which the purified Rnf complex of the Acetobacterium woodii bacterium was “shock-frozen” and then dripped onto a carrier plate.
A thin film of ice is created in the process, which contains millions of Rnf complexes that can be observed using an electron microscope. Since they fall onto the carrier plate differently during the dripping process, it is possible to see different sides of them under the microscope.
“These images can be combined into a three-dimensional one, which gave us a precise insight into the structure of the complex – especially those parts that are essential to the transfer of electrons,” Kumar explains. The analysis of images taken at different intervals shows that far from being rigid, the individual components of the complex move back and forth dynamically. This allows the electron carriers to bridge longer distances and pass on their cargo.
Fundamentally new mechanism
The question remained: How does the flow of electrons drive the outflow of sodium ions? A molecular dynamics simulation by Prof. Dr. Ville Kaila’s working group at Stockholm University provided an initial answer to this question. A key role is played by a cluster of iron and sulphur atoms located in the middle of the membrane, which, after picking up an electron, becomes negatively charged.
“The positively charged sodium ions from inside the cell are drawn to this cluster, just like a magnet,” explains Jennifer Roth, a doctoral candidate in Müller’s research group. “This attraction in turn causes the proteins to shift around the iron-sulphur cluster, much like a rocker switch: they create an opening leading to the outside of the membrane, through which the sodium ions are once again released.”
Roth was able to confirm this process by making specific genetic changes to the Rnf proteins. The fact that this fundamentally new mechanism could be elucidated is a testament to the successful cooperation between the three universities. Making the results even more interesting is the microorganisms’ ability to absorb CO2 from their environment during the acetic acid production process.
This ability could potentially be used to remove greenhouse gases from industrial waste emissions, for example. It could help slow down climate change while simultaneously providing valuable starting materials for the chemical industry.
“Once we know how the bacteria generate energy in the process, we may be able to optimize this process in a manner that would allow us to produce even higher-quality end products,” is Müller’s hope. The findings could also provide starting points for new drugs against pathogens with similar respiratory enzymes.
Journal Reference:
Kumar, A., Roth, J., Kim, H. et al., ‘Molecular principles of redox-coupled sodium pumping of the ancient Rnf machinery’, Nature Communications 16, 2302 (2025). DOI: 10.1038/s41467-025-57375-8
Article Source:
Press Release/Material by Goethe University Frankfurt
Featured image credit: David Bartus | Pexels