Explore the latest insights from top science journals in the Muser Press daily roundup, featuring impactful research on climate change challenges.
In brief:
Climate change threatens global cocoa production
Cocoa (Theobroma cacao L.) is a vital cash-crop for four to six million small-holder farmers across the tropics, and supports a global chocolate industry valued at over USD 100 billion annually. The combination of millions of farmers relying on cocoa for their livelihoods, and increasing global demand for the crop, has driven cocoa plantation expansion and intensification of farming practices, often at the expense of biodiversity and long-term sustainability.

A new study led by the University of Oxford, in collaboration with Westlake University, China, Universidade Estadual de Santa Cruz, Brazil, and University of Göttingen, Germany, has highlighted the significant risks posed to cocoa production by climate change. However, the authors also identified farm management solutions that can both climate-proof cocoa crops and boost productivity without the need to expand plantations into forests.
The research, conducted across three major cocoa-producing countries — Brazil, Ghana, and Indonesia, which together account for 33% of global cocoa production — investigated key factors influencing cocoa yields. The findings revealed that increasing pollination rates above current levels could boost yields by 20%. This demonstrates that insufficient pollination is occurring to produce the maximum possible yield for many cocoa plantations. Separate to the impact of pollination, sites where temperatures were up to 7 degrees warmer had 20-31% lower cocoa yields, underscoring the vulnerability of cocoa-producing regions to the effects of climate change.
The study has been published in Communications Earth & Environment.
Co-author Dr Acheampong Atta-Boateng, who recently completed his doctoral work at the University of Oxford, said: ‘Cocoa is pollinated by tiny insects such as midges and thrips, and it comes as quite a surprise that most of the time there simply isn’t enough pollination happening to produce the cocoa crop that is possible.’
To support sustainable cocoa production, researchers recommend practical strategies to enhance pollination, such as maintaining leaf litter and other understory biomass, preserving soil organic matter, providing moderate shade, and reducing agricultural chemical use. These practices not only increase pollinator abundance, but also help regulate plantation temperatures and improve soil health, ensuring long-term plantation resilience.
Dr Tonya Lander, from the University of Oxford and first author of the study said: “This research shows that sustainable agricultural methods can significantly improve cocoa yields without farm expansion or intensification. By adopting biodiversity-centred, climate-resilient farming techniques, the cocoa sector can both increase production and safeguard farmers’ livelihoods.”
Dr Tom Wanger of Westlake University, China added: “The rising demand for cocoa and the short-term economic benefits to farmers has led to plantation expansion and ecological homogenization at the expense of biodiversity and vital ecosystem services, like pollination. This study highlights the long-term risks of this approach, and how pollination can be a solution that works alongside climate-resilient agricultural systems to achieve long-term, ecologically and financially sustainable solutions.”
Journal Reference:
Lander, T.A., Atta-Boateng, A., Toledo-Hernández, M. et al., ‘Global chocolate supply is limited by low pollination and high temperatures’, Communications Earth & Environment 6, 97 (2025). DOI: 10.1038/s43247-025-02072-z
Article Source:
Press Release/Material by University of Oxford
New 2.5-dimensional skeletons in porous organic crystals are key to superior CO2 separation
Porous organic crystals with superior properties as CO2 adsorbents were created by researchers at Institute of Science Tokyo. Owing to the novel 2.5-dimensional skeleton, the materials feature ultrahigh-density amines. The covalently-bonded microporous skeleton and high crystallinity realize fast CO2 adsorption and high thermal stability. Their low adsorption heat, only one-fourth of the current amine scrubbing method, and their light-elemental nature can reduce the cost for CO2 separation from flue gases.
To mitigate climate change, CO2 emissions from large industrial facilities have to be reduced. To separate CO2 from the flue gases, the major current technology is the amine scrubbing method, in which an aqueous solution of amine molecules is circulated in the capture facility to cyclically perform capture and release of CO2.
However, this method suffers from reportedly high running costs and several problematic aspects of amine solutions such as high environmental risk and corrosivity to steels.
The high cost arises from the necessity to also heat up the liquid water, which is a solvent of the amine molecules, and to generate steam that requires a large energy input to generate, in the regeneration process to strip the captured CO2 from the amine molecules. The high cost is also caused by the excessively large heat of reaction (denoted Q; typically, in 80-100 kJ/mol range).
Therefore, to avoid the high cost, one should cease the use of aqueous amine solution and (2) decrease Q as low as possible. To achieve the first, the use of solid sorbents is reasonable. However, if we use non-porous solids, the capturing speed would be intolerably slow. Thus, we should use porous solids. The use of solid sorbents would also resolve the issues of corrosion and environmental risk.
Achieving the second is more difficult, because a high Q assures the speed of CO2 capture and high selectivity to CO2 over other species in air like nitrogen and oxygen. In other words, Q at a level that is too low will cause an intolerably low CO2 capturing speed and low selectivity to CO2. Therefore, to decrease the cost, we have to decrease Q without scarifying the CO2 capturing speed and selectivity to CO2. That is the technical dilemma.
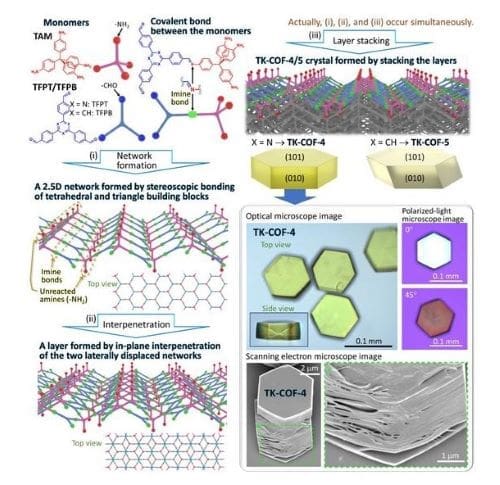
The organic porous materials recently reported by the researchers of Institute of Science Tokyo (Science Tokyo) have a slightly peculiar structure. Initially, the researchers were trying to polymerize two kinds of monomers, which are a tetrahedral molecule with four primary amines (-NH2) on the vertexes (TAM) and a triangle molecule with aldehydes (-CHO) on the vertexes (TFPT/TFPB), as shown in the upper left portion of Figure 1.
Here, -NH2 and -CHO serve as “hands” to form a covalent bond; a “shaking-of-hands” between -NH2 and -CHO is known to form an imine bond (-HN=C-), a type of covalent bond, with a release of one H2O molecule. The researcher thought that they would obtain solids with granular shapes as a result of a three-dimensional (3D) network formation, as geometrically expected from the “four hands” of the tetrahedron monomer. In short, they planned to generate 3D covalent organic frameworks (3D-COFs).
However, their expectation did not come true. Instead, they obtained two-dimensional (2D) solids composed of stacked layers, as shown in the bottom right portion of Figure 1. The morphology is similar to graphite that is composed of stacked layers of graphene (an atomic layer of hexagonally-bonded carbon atoms). The researchers were puzzled by this morphology, which is a typical morphology of 2D covalent organic frameworks (2D-COFs).
The answer was revealed by the single-crystal X-ray diffraction analysis. The layered solids were composed of corrugated framework layers constructed with imine bonds of 3D connectivity, ending up in a macroscopically two-dimensionally extended polymer for one layer (Figure 1).
The stacking of the layers resulted in the formation of the layered solids. Because such a framework structure was unexpected from the geometry of the monomers and did not match previous depictions or definitions of 2D-COFs nor 3D-COFs, the researchers thought it would be appropriate to call such materials 2.5-dimensional COFs (2.5D-COFs).
Because this structure was constructed using only three hands of a tetrahedral monomer, one hand is left unused per the monomer in the material (Figure 1). Consequently, the material uniquely has ultrahigh density amine (-NH2) moieties, where all -NH2 are regularly arrayed and pointing normal to the 2D layer. This material is microporous (pore size: 6-7 Å) and -NH2 is the moiety that has, more or less, an ability to capture CO2 molecules.
Project leader Professor Yoichi Murakami commented: “Although that structure was anticipated when I first looked at the layered morphology, I was excited when the results of the single-crystal X-ray diffraction analysis actually exhibited such an unprecedented network structure. Noticing such an ultrahigh-density array of primary amines in these materials, our group soon decided to investigate the CO2 adsorption properties. The properties were very good, as we anticipated.”
In their study published in the journal Nature Communications, the researchers found that the heat of adsorption, Q, was much lower (about 25 kJ/mol) than that in the amine scrubbing method (typically 80-100 kJ/mol) and other porous organic materials. Importantly, these 2.5D-COFs do not suffer from the aforementioned dilemma. Despite their significantly low Q, they exhibited sufficiently high selectivity to CO2 over N2 (100 or higher) and a high speed of CO2 adsorption (equilibrium time constant < 10 s). They have high thermal stability in air to around 300 °C.
Simultaneous achievements of these advantages render these 2.5D-COFs promising materials that can efficiently capture and separate CO2 at a lower cost than the current technology, whether the separated CO2 is reused or buried underground, ultimately to mitigate climate change.
Journal Reference:
Tomoki Kitano, Syunto Goto, Xiaohan Wang, Takayuki Kamihara, Yoshihisa Sei, Yukihito Kondo, Takumi Sannomiya, Hidehiro Uekusa & Yoichi Murakami, ‘2.5-dimensional covalent organic frameworks’, Nature Communications 16, 280 (2025). DOI: 10.1038/s41467-024-55729-2
Article Source:
Press Release/Material by Institute of Science Tokyo
The opportunity costs of carbon capture
For most countries around the world, sourcing energy entirely from wind, solar, geothermal, and hydropower by 2050 would reduce their energy needs and costs, improve air quality, and help slow climate change, according to a study published in Environmental Science & Technology.
These benefits, the authors say, could be realized at a fraction of the cost of implementing technologies that remove carbon dioxide (CO2) from the air and capture it from stationary emitters like industrial smokestacks.
“If you spend $1 on carbon capture instead of on wind, water, and solar, you are increasing CO2, air pollution, energy requirements, energy costs, pipelines, and total social costs,” said lead study author Mark Jacobson, a professor of civil and environmental engineering in the Stanford Doerr School of Sustainability and Stanford School of Engineering.
This holds true even if zero-emission energy systems power the technology deployed to extract carbon dioxide, Jacobson added. “It’s always an opportunity cost to use clean, renewable energy for direct air capture instead of replacing a fossil-fuel CO2 source, just like it’s an opportunity cost to use it for AI or bitcoin mining. You’re preventing renewables from replacing fossil fuel sources because you’re creating more demand for those renewables,” he said.

Comparing two extremes
Jacobson and co-authors compared the annual energy costs, emissions, public health impacts, and social costs associated with implementing either of two extremes across all sectors in 149 countries over the next 25 years.
One extreme would see a complete switch to use heat and electricity generated by wind, solar, geothermal, and hydropower for all energy needs, as well as some advances in energy efficiency; cuts to energy demand through improved public transit, increased biking, and telecommuting; and commercialization of hydrogen fuel cells for long-distance air travel and shipping. For this case, the researchers assume hydrogen would be produced using water and electricity from renewable sources, not with fossil fuels, which is the way most hydrogen is made today.
The other extreme would see countries maintain their current reliance on fossil fuels with some renewables, nuclear, and biomass – while improving energy efficiency by the same amount as in the all-renewable case. In this second extreme, all 149 countries would also add equipment to capture carbon dioxide from industrial flues and use technology known as synthetic direct air carbon capture to pull CO2 from ambient air.
Comparing these two “unrealistically extreme cases,” the authors write, distills the climate, health, and social costs associated with investing money in carbon capture and direct air capture that might otherwise go toward electrification and wind, water, and solar power. Neither case considers the potential costs or benefits of efforts to enhance carbon sequestration in natural carbon sinks like wetlands, forests, soil, and oceans.
Benefits of eliminating combustion
Jacobson and co-authors found that if the 149 studied countries successfully eliminated fossil fuels and biomass combustion through renewables and efficiency gains by 2050, they could reduce their end-use energy needs by more than 54%. Annual energy costs, the authors concluded, would decline by nearly 60%. Hundreds of millions of illnesses and 5 million deaths per year related to air pollution from energy – whether from woodburning cookstoves and kerosene lamps or from gas-fired power stations – would be avoided.
“When you add wind turbines to replace a coal plant, you’re eliminating not only the CO2 but also the pollution from the coal,” said Jacobson, who is also a senior fellow at the Stanford Woods Institute for the Environment.
Widespread electrification reduces energy demand in part because electric heat pumps and vehicles are more efficient than gas heaters and appliances, conventional air conditioners, and internal combustion engines, Jacobson said. Other energy savings come from eliminating energy needed to extract, transport, and refine oil, gas, coal, and uranium.
“You can have the most efficient way of removing CO2 from the air, but that does not change the efficiency of combustion. You’re keeping that inefficient energy infrastructure the same,” said Jacobson. “It’s much cheaper and more efficient just to replace the fossil source with electricity or heat provided by a renewable source.”
Climate policies that promote expansion of renewables as well as carbon capture and direct air capture to deal with emissions from fossil fuels and biomass “do not distinguish between good and poor solutions,” and any policy promoting carbon capture and direct air capture “should be abandoned,” the authors write in the study. They add, “The only way to eliminate all air-pollutant and climate-warming gases and particles from energy is to eliminate combustion.”
Journal Reference:
Mark Z. Jacobson, Danning Fu, Daniel J. Sambor, and Andreas Mühlbauer, ‘Energy, Health, and Climate Costs of Carbon-Capture and Direct-Air-Capture versus 100%-Wind-Water-Solar Climate Policies in 149 Countries’, Environmental Science & Technology . DOI: 10.1021/acs.est.4c10686
Article Source:
Press Release/Material by Josie Garthwaite | Stanford Doerr School of Sustainability
Featured image credit: Gerd Altmann | Pixabay




