As the global agriculture industry seeks sustainable solutions to reduce greenhouse gas emissions, scientists are finding ways to decrease reliance on nitrogen-based fertilizers.
In a study published in New Phytologist, researchers investigated how manipulating a specific protein in potatoes might reduce the crop’s nitrogen needs, paving the way for more eco-friendly farming practices.
Nitrogen fertilizers, widely used in modern agriculture, contribute significantly to greenhouse gas emissions, making nitrogen-efficient crops a priority for sustainable food production.
Originally native to the Andes, potatoes developed a survival strategy of growing tubers in winter to store nutrients, responding to the shorter daylight hours as a cue. However, when potatoes were brought to Europe in the 16th century, they faced a new challenge: the shorter winter days coincided with freezing temperatures, which killed the plants before they could produce large tubers.
Over time, a natural mutation in the StCDF1 gene, which regulates tuber growth, allowed potatoes to adapt, enabling tuber formation regardless of day length. This mutation ultimately helped potatoes thrive in northern climates without relying on seasonal cues.
Researchers discovered in their study that StCDF1 also influences nitrogen-related gene expression in the plant. This connection suggests a possible path to cultivating potatoes that thrive in low-nitrogen conditions. According to Salomé Prat, a co-corresponding author of the study and a research professor at Spain’s Centre for Research in Agricultural Genomics, the results are promising.
“Natural variation in StCDF1 binding to the single potato NITRATE REDUCTASE gene emerges as a promising strategy to reduce potato needs of nitrogen fertilizers, as this gene encodes a limiting step for nitrate reduction and later assimilation,” Prat said.
Nitrogen is vital for plant growth, forming the basis of amino acids, proteins, and chlorophyll. In potatoes, the uptake and assimilation of nitrogen are tightly regulated, involving complex interactions among different gene families and proteins that process nitrate in various stages.
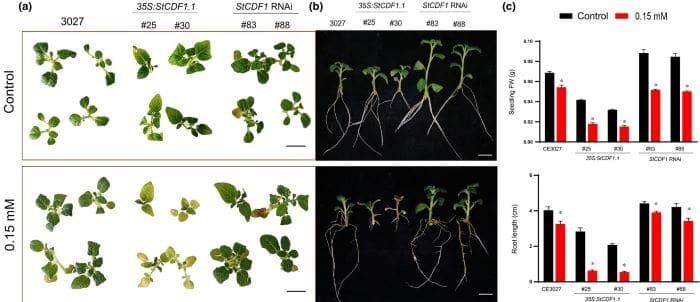
The study’s authors analyzed StCDF1’s molecular targets through DNA-binding techniques and combined this data with transcriptomic analyses of potato variants modified to either overexpress or suppress StCDF1. Results confirmed that altering StCDF1 expression significantly impacts nitrogen metabolism, specifically by down-regulating the NITRATE REDUCTASE gene in potatoes. This modulation allowed for improved growth and yield in nitrogen-deficient soil conditions.
One challenge in developing nitrogen-efficient potatoes has been the crop’s naturally high demand for nitrogen to produce maximum yields, often coupled with shallow root systems that make nutrient uptake less efficient. However, as Prat and her team demonstrated, blocking StCDF1 can mitigate some of these limitations.
The findings also align with previous studies in model plants like Arabidopsis and rice, where similar regulatory mechanisms were observed. The NIN-LIKE PROTEIN 7 (NLP7) family, another nitrogen-sensitive gene regulator, shares overlapping functions with StCDF1, suggesting broader applications of this research in other nitrogen-hungry crops.
The study’s insights offer potential pathways for enhancing agricultural sustainability by genetically adjusting nitrogen use efficiency in major staple crops. If commercialized, nitrogen-efficient potato strains could reduce reliance on synthetic fertilizers, thereby lowering costs for farmers and decreasing environmental impact. As the agriculture sector grapples with climate-related challenges, such advancements are crucial for promoting sustainable food systems globally.
Journal Reference:
Maroof Ahmed Shaikh, Lorena Ramírez-Gonzales, José M. Franco-Zorrilla, Evyatar Steiner, Marian Oortwijn, Christian W. B. Bachem, Salomé Prat, ‘StCDF1: A ‘jack of all trades’ clock output with a central role in regulating potato nitrate reduction activity’, New Phytologist (2024). DOI: 10.1111/nph.20186
Article Source:
Press Release/Material by Wiley
Featured image credit: Fayette Reynolds M.S. | Pexels