Researchers from Tokyo Metropolitan University have developed a cutting-edge electrochemical cell that could revolutionize the conversion of captured carbon dioxide (CO2) into green fuel.
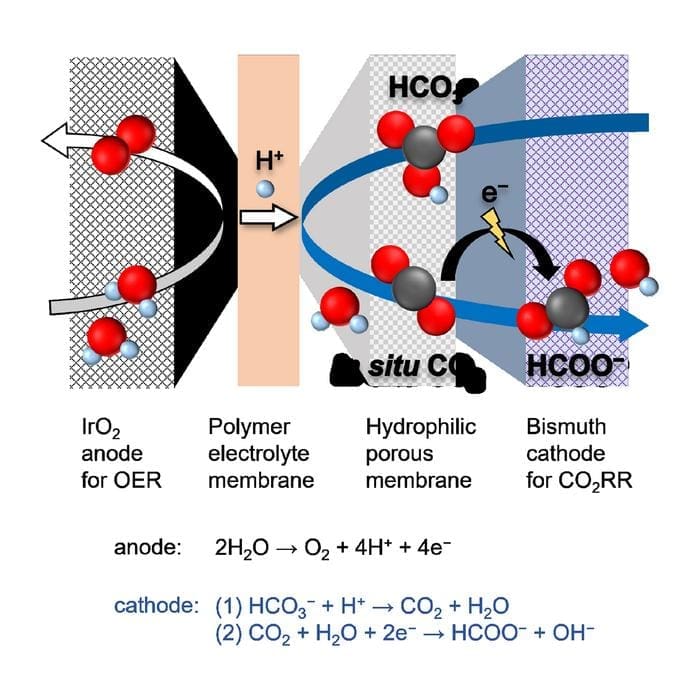
Their novel design converts bicarbonate solutions derived from captured CO2 into formate, a valuable green fuel, with impressive efficiency. This innovation marks a key step toward industrial-scale carbon utilization, addressing challenges in reactive carbon capture (RCC) and rivaling the performance of energy-intensive gas-fed methods.
Carbon capture plays a crucial role in global efforts to reduce emissions and mitigate climate change. However, the question of how to effectively use captured CO2 remains unresolved. While storing CO2 underground is one option, scientists are seeking ways to convert this waste into useful products.
Among the most promising applications is the conversion of CO2 into formate, a compound that can be used in fuel cells to generate clean energy.
Previous attempts at converting CO2 into formate faced challenges, particularly the need for pure CO2, which is energy-intensive to produce and convert. Reactive carbon capture offers an alternative by utilizing CO2 dissolved in alkaline solutions, like bicarbonate. However, researchers needed a more efficient electrochemical cell to selectively convert bicarbonate into formate without unwanted side reactions.
The team led by Professor Fumiaki Amano has overcome these hurdles by developing a new cell with a porous cellulose ester membrane.
This design enables highly selective production of formate ions, with a faradaic efficiency of 85%, even under high currents. Moreover, the cell operates for over 30 hours with nearly complete conversion of bicarbonate to formate, leaving behind solid, crystalline fuel once the water is removed.
This breakthrough has the potential to significantly enhance the efficiency of CO2 conversion technology, directly adding value to carbon waste streams. The researchers hope their new bicarbonate electrolyzer will contribute to the global shift toward net-zero emissions.
***
The work was supported by the Tokyo Metropolitan Government.
Journal Reference:
Kohta Nomoto, Takuya Okazaki, Kosuke Beppu, Tetsuya Shishido and Fumiaki Amano, ‘Highly selective formate formation via bicarbonate conversions’, EES Catalysis (2024). DOI: 10.1039/D4EY00122B
Article Source:
Press Release/Material by Tokyo Metropolitan University
Featured image credit: Freepik




