Scientists at ETH Zurich have made significant strides in chemical recycling, a process poised to transform plastic waste management by producing high-quality products from discarded materials.
Their novel work has demonstrated the ability to break down common plastics like polyethylene and polypropylene into molecules that can be repurposed as fuels or lubricants. This development sets new standards for the global scientific community, providing the necessary foundation to advance chemical recycling technology.
The traditional approach to recycling plastic waste – mechanical recycling – involves shredding and melting plastics, which unfortunately leads to a deterioration in product quality with each cycle. Chemical recycling, however, offers a promising alternative by breaking down plastic polymers into their fundamental monomers. These can be reassembled into new, high-quality plastics, supporting a true circular economy.
The team at ETH Zurich, led by Professor Javier Pérez-Ramírez, focused on converting long-chain polymers into shorter molecules suitable for use as liquid fuels, such as petrol or jet fuel. Their research involved melting plastics in a steel tank and introducing hydrogen gas into the molten material.
The critical innovation came from their use of a powdered catalyst containing metals like ruthenium, which significantly enhanced the chemical reaction’s efficiency, producing specific molecular chain lengths while minimizing unwanted byproducts.
One of the key challenges in this process is ensuring the effective mixing of the thick, honey-like molten plastic with the catalyst and hydrogen. Researcher Antonio José Martín highlighted the importance of stirrer geometry and speed in achieving optimal results: “The key is how you stir it in the tank to ensure the catalyst powder and hydrogen get mixed right through.”
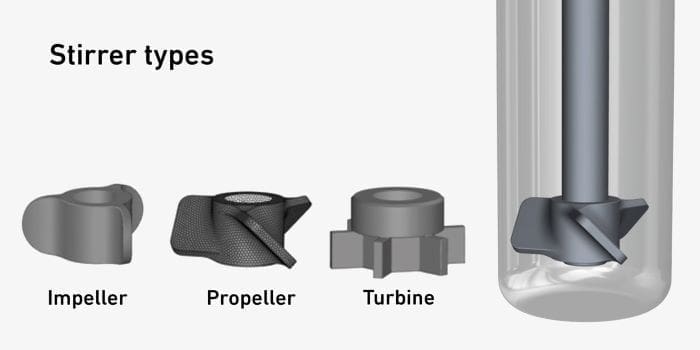
The team discovered that using an impeller with blades parallel to the axis – rather than angled blades or a turbine – produced the most consistent mixing, reducing flow vortices. They also determined that a stirring speed of around 1,000 revolutions per minute was ideal.
The researchers’ success culminated in the development of a mathematical formula that models the entire chemical recycling process, accounting for all relevant parameters. This formula is a crucial tool for future experiments, enabling precise comparisons of different catalysts under controlled conditions. It also holds significant potential for scaling up the technology from laboratory settings to industrial recycling plants.
While the ETH Zurich team’s focus remains on refining catalysts to improve the efficiency of chemical recycling, their work represents a major leap forward in the field. By providing the scientific community with new tools and insights, they have laid the groundwork for a more sustainable approach to plastic waste management.
***
This research was supported by NCCR Catalysis, a National Centre of Competence in Research.
Journal Reference:
Jaydev, S.D., Martín, A.J., Garcia, D., Chikri, K., Pérez-Ramírez, J. ‘Assessment of transport phenomena in catalyst effectiveness for chemical polyolefin recycling’, Nature Chemical Engineering (2024). DOI: 10.1038/s44286-024-00108-3
Article Source:
Press Release/Material by ETH Zurich
Featured image: Most drink bottle caps are made of polypropylene. Along with polyethylene they account for 60 percent of all plastic waste. Credit: Shibashish Jaydev | ETH Zurich




